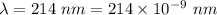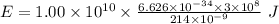Chemistry, 30.10.2019 03:31 lilyella1004
Part a the emission line used for zinc determinations in atomic emission spectroscopy is 214 nm. if there are 1.00×1010 atoms of zinc emitting light in the instrument flame at any given instant, what energy (in joules) must the flame continuously supply to achieve this level of emission? express your answer numerically in joules.
part b during an emission, electrons move from a higher energy orbital to a lower energy orbital. which of the following are valid transitions that produce lines in the emission spectrum of zn?
check all that apply.
1-[ar]4s13d106s1→[ar]4s23d10
2-[ar]4s23d10→[ar]4s23d104p2
3-[ar]4s23d10→[ar]3d10
4-[ar]4s23d10→[ar]4s13d11
5-[ar]3d10→[ar]4s23d10
6-[ar]4s23d10→[ar]4s13d106s1

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

You know the right answer?
Part a the emission line used for zinc determinations in atomic emission spectroscopy is 214 nm. if...
Questions


Biology, 12.08.2020 04:01




Mathematics, 12.08.2020 04:01

Biology, 12.08.2020 04:01

Social Studies, 12.08.2020 04:01


History, 12.08.2020 04:01

Social Studies, 12.08.2020 04:01


Mathematics, 12.08.2020 04:01




Physics, 12.08.2020 04:01

Mathematics, 12.08.2020 04:01

Physics, 12.08.2020 04:01

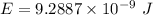
![[Ar]4s^13d^{10}6s^1\rightarrow [Ar]4s^23d^{10}](/tpl/images/0352/1669/c1431.png)
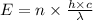
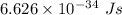
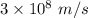
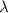 is the wavelength
is the wavelength