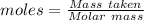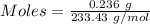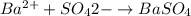Chemistry, 30.10.2019 03:31 putaprincess16
Asolution contains an unknown mass of dissolved barium ions. when sodium sulfate is added to the solution, a white precipitate forms. the precipitate is filtered and dried and then found to have a mass of 236 mg. what mass of barium was in the original solution? (assume that all of the barium was precipitated out of solution by the reaction.)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Clouds form when water vapor to form small droplets. a. humidifies b. condenses c. evaporates d. precipitates
Answers: 2

Chemistry, 21.06.2019 19:20
What is the strongest intermolecular force between an nacl unit and an h2o molecule together in a solution? covalent bonding dipole-dipole force hydrogen bonding ion-dipole force
Answers: 1

Chemistry, 21.06.2019 19:30
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1

You know the right answer?
Asolution contains an unknown mass of dissolved barium ions. when sodium sulfate is added to the sol...
Questions


Mathematics, 05.11.2020 21:40



Mathematics, 05.11.2020 21:40

Arts, 05.11.2020 21:40




Mathematics, 05.11.2020 21:40


Mathematics, 05.11.2020 21:40

Mathematics, 05.11.2020 21:40


Mathematics, 05.11.2020 21:40

Mathematics, 05.11.2020 21:40

Biology, 05.11.2020 21:40

Mathematics, 05.11.2020 21:40


Chemistry, 05.11.2020 21:40

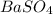 obtained on precipitation = 236 mg
obtained on precipitation = 236 mg