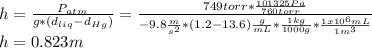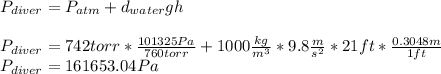Chemistry, 30.10.2019 03:31 jpichardo2021
The compound 1-iodododecane is a nonvolatile liquid with a density of 1.20g/ml. the density of mercury is 13.6g/ml. what do you predict for the height of a barometer column based on 1-iodododecane, when the atmospheric pressure is 749 torr? what is the pressure, in atmospheres, on the body of a diver if he is 21 ft below the surface of the water when the atmospheric pressure is 742 torr?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 21.06.2019 22:00
Bohr's model could only explain the spectra of which type of atoms? single atoms with one electron single atoms with more than one electron bonded atoms with one electron bonded atoms with more than one electron
Answers: 2

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1
You know the right answer?
The compound 1-iodododecane is a nonvolatile liquid with a density of 1.20g/ml. the density of mercu...
Questions






Mathematics, 25.07.2019 04:30


Mathematics, 25.07.2019 04:30

Mathematics, 25.07.2019 04:30



Mathematics, 25.07.2019 04:30



English, 25.07.2019 04:30

Mathematics, 25.07.2019 04:30

Mathematics, 25.07.2019 04:30

Mathematics, 25.07.2019 04:30


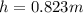
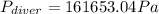
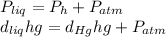
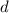 is the density,
is the density, 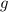 the acceleration of gravity,
the acceleration of gravity, 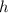 the height and
the height and 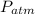 the atmospheric pressure, thus:
the atmospheric pressure, thus: