
Lemon juice has a ph of about 2.5. assuming that the acidity of lemon juice is due solely to citric acid, that citric acid is a monoprotic acid, and that the density of lemon juice is 1.0 g/ml, then the citric acid concentration calculates to 0.5% by mass. estimate the volume of 0.0100 m naoh required to neutralize a 3.71-g sample of lemon juice. the molar mass of citric acid is 190.12 g/mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3
You know the right answer?
Lemon juice has a ph of about 2.5. assuming that the acidity of lemon juice is due solely to citric...
Questions

Mathematics, 30.11.2020 22:00



Mathematics, 30.11.2020 22:00


Biology, 30.11.2020 22:00

Mathematics, 30.11.2020 22:00



Mathematics, 30.11.2020 22:00

Chemistry, 30.11.2020 22:00


History, 30.11.2020 22:00






History, 30.11.2020 22:00

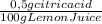 = 0,01855 g of citric acid. In moles:
= 0,01855 g of citric acid. In moles: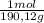 = 9,76x10⁻⁵ moles of citric acid.
= 9,76x10⁻⁵ moles of citric acid.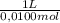 = 9,76x10⁻³L ≡ 9,76 mL
= 9,76x10⁻³L ≡ 9,76 mL

