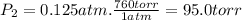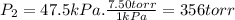Chemistry, 30.10.2019 01:31 alexmoy45p8yd7v
Three glass bulbs, joined by closed stopcocks, have the following volumes and initial pressures of the specified gases. bulb a: 150. ml of co(g) at 190. torr bulb b: 300. ml of ar(g) at 0.500 atm bulb c: 750. ml of kr(g) at 75.994 kpa 1. after both stopcocks are opened and the gases allowed to diffuse throughout, what will be the ultimate total pressure? 2. what is the partial pressure of co(g)?
3. what is the mole fraction of co₂(g)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 22.06.2019 04:30
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1
You know the right answer?
Three glass bulbs, joined by closed stopcocks, have the following volumes and initial pressures of t...
Questions

World Languages, 07.01.2020 17:31

Mathematics, 07.01.2020 17:31


Biology, 07.01.2020 17:31

Mathematics, 07.01.2020 17:31



Biology, 07.01.2020 17:31

History, 07.01.2020 17:31


Mathematics, 07.01.2020 17:31


History, 07.01.2020 17:31


Physics, 07.01.2020 17:31

Health, 07.01.2020 17:31

Biology, 07.01.2020 17:31



Mathematics, 07.01.2020 17:31