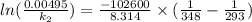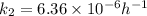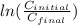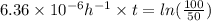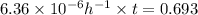Chemistry, 30.10.2019 00:31 qudoniselmore0
The hydrolysis of pyrophosphate can be described by the reaction h2po2 7 +h2o ! 2h2po 4 if the ph is held constant, the reaction is pseudo-first order with respect to h2po2– 7 . 126 (a) if, at a given ph, the half-life of pyrophosphate is 140 h at 75c and 13 h at 100c, what is ear for the reaction? (b) estimate the time required for 50% hydrolysis of pyrophosphate in a solution at the same ph as in part (a), but at 20c.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1
You know the right answer?
The hydrolysis of pyrophosphate can be described by the reaction h2po2 7 +h2o ! 2h2po 4 if the ph i...
Questions

Mathematics, 30.10.2020 17:40

History, 30.10.2020 17:40

Social Studies, 30.10.2020 17:40

Mathematics, 30.10.2020 17:40

Mathematics, 30.10.2020 17:40


Chemistry, 30.10.2020 17:40









Mathematics, 30.10.2020 17:40


Health, 30.10.2020 17:40


Chemistry, 30.10.2020 17:40

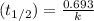
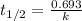
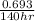
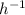
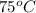 is 0.00495
is 0.00495 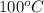 , value of rate constant will be as follows.
, value of rate constant will be as follows.
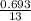
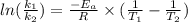
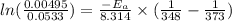
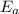 = 102.6 kJ/mol
= 102.6 kJ/mol
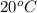 ,
,