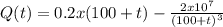Chemistry, 30.10.2019 00:31 lamashermosa23
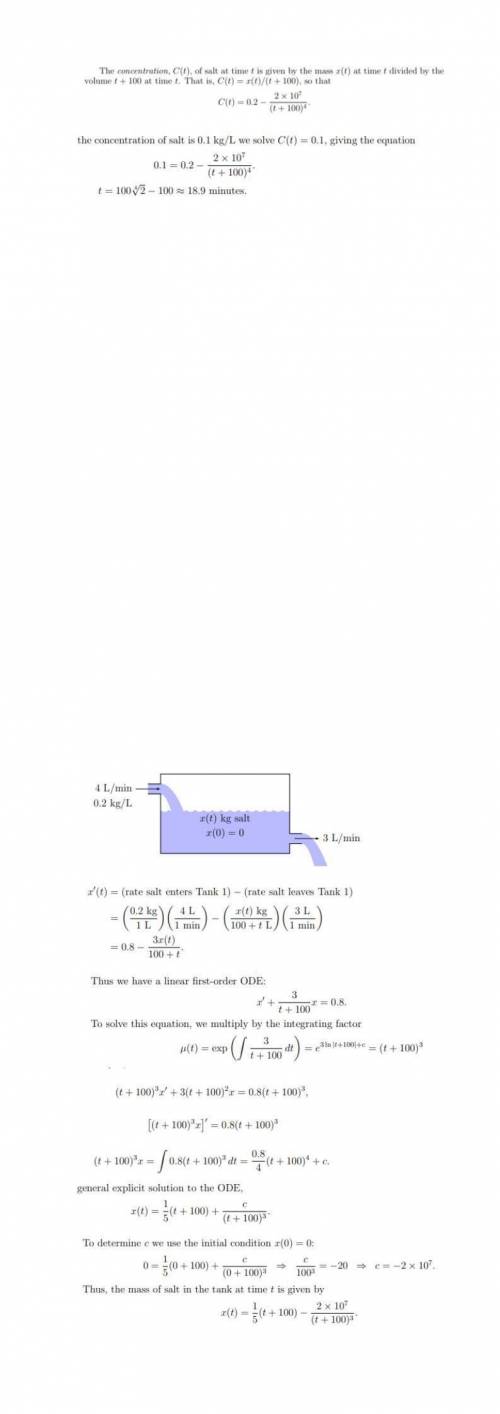
Abrine solution of salt flows at a constant rate of 4l/min into a large tank that initially held 100l of pure water. the solution inside the tank is kept well-stirred and flows out of the tank at a rate of 3l/min. if the concentration of salt in the brine entering the tank is 0.2kg/l, determine the mass of the salt in the tank after t minutes. when will the concentration of salt in the tank reach 01.kg/l?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Zinc + lead(ii) nitrate yield zinc nitrate + leadwhat's the chemical equation for this?
Answers: 1

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1
You know the right answer?
Abrine solution of salt flows at a constant rate of 4l/min into a large tank that initially held 100l...
Questions

Mathematics, 03.04.2020 07:59

History, 03.04.2020 07:59


History, 03.04.2020 07:59


Mathematics, 03.04.2020 07:59



Mathematics, 03.04.2020 07:59

English, 03.04.2020 07:59


Mathematics, 03.04.2020 07:59



Mathematics, 03.04.2020 08:00

Business, 03.04.2020 08:00


History, 03.04.2020 08:00