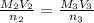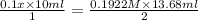Chemistry, 30.10.2019 00:31 tyijiapostell
Achemist needs to determine the concentration of a sulfuric acid solution by titration with a standard sodium hydroxide solution. he has a 0.1922 m standard sodium hydroxide solution. he takes a 25.00 ml sample of the original acid solution and dilutes it to 250.0 ml. then, he takes a 10.00 ml sample of the dilute acid solution and titrates it with the standard solution. the endpoint was reached after the addition of 13.68 ml of the standard solution. what is the concentration of the original sulfuric acid solution?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which traits do human embryos have that link them to a common ancestor with fish and reptiles? a. scales and tail b. gill slits and scales c. tail and gill slits d. hair and tail
Answers: 2

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1


Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1
You know the right answer?
Achemist needs to determine the concentration of a sulfuric acid solution by titration with a standa...
Questions

English, 18.09.2021 09:00

Biology, 18.09.2021 09:00

Law, 18.09.2021 09:00

Mathematics, 18.09.2021 09:00

Physics, 18.09.2021 09:00


Mathematics, 18.09.2021 09:00

English, 18.09.2021 09:00

Social Studies, 18.09.2021 09:00

Physics, 18.09.2021 09:00


Mathematics, 18.09.2021 09:10

Business, 18.09.2021 09:10


Chemistry, 18.09.2021 09:10


Mathematics, 18.09.2021 09:10


Mathematics, 18.09.2021 09:10

Chemistry, 18.09.2021 09:10

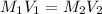
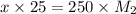
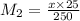
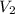 = 10.00 mL (given)
= 10.00 mL (given)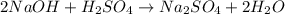
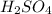 = Concentration × Volume of NaOH
= Concentration × Volume of NaOH