
Ammonia (nh3) ionizes according to the following reaction: nh3(aq) + h2o(l) ⇌ nh4+(aq) + oh–(aq) the base dissociation constant for ammonia (nh3) is kb = 1.8 × 10–5. ammonia (nh3) also has a chloride salt, ammonium chloride (nh4cl), which is soluble in water. if 0.070 m of ammonia (nh3) and 0.035 m of its salt ammonium chloride (nh4cl) are mixed in a solution, what is the ph of this solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
Ammonia (nh3) ionizes according to the following reaction: nh3(aq) + h2o(l) ⇌ nh4+(aq) + oh–(aq) th...
Questions



Mathematics, 29.10.2019 10:31


Biology, 29.10.2019 10:31


English, 29.10.2019 10:31

Mathematics, 29.10.2019 10:31



English, 29.10.2019 10:31


Mathematics, 29.10.2019 10:31


Biology, 29.10.2019 10:31



Mathematics, 29.10.2019 10:31

History, 29.10.2019 10:31

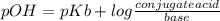
![pOH = pKb + log\frac{[NH_{4}^{+} ]}{[NH_{3}]} =4.7+log\frac{0.035M}{0.070M} =4.4](/tpl/images/0351/7427/0637f.png)


