
Chemistry, 29.10.2019 21:31 Adolfosbaby
A0.5222 g solid sample containing a mixture of lacl3 (molar mass = 245.26 g/mol) and ce(no3)3 (molar mass = 326.13 g/mol) was dissolved in water. the solution was titrated with kio3 , producing the precipitates la(io3)3 and ce(io3)3 . for the complete titration of both la3+ and ce3+ , 42.81 ml of 0.1250 m kio3 was required. calculate the mass fraction of la and ce in the sample.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:40
In the reading, yao chen-yuan describes traveling to deliver a message. why was he willing to risk danger to travelto tientsin? he wanted to the boxers with their cause
Answers: 2

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2


Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1
You know the right answer?
A0.5222 g solid sample containing a mixture of lacl3 (molar mass = 245.26 g/mol) and ce(no3)3 (molar...
Questions


History, 03.12.2020 21:00

Mathematics, 03.12.2020 21:00


History, 03.12.2020 21:00

History, 03.12.2020 21:00

Mathematics, 03.12.2020 21:00


History, 03.12.2020 21:00


Mathematics, 03.12.2020 21:00

History, 03.12.2020 21:00

Mathematics, 03.12.2020 21:00

English, 03.12.2020 21:00


Mathematics, 03.12.2020 21:00


Mathematics, 03.12.2020 21:00


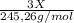 +
+ 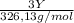 (1)
(1)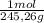 ×
×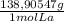 = 0,1022g of La
= 0,1022g of La 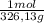 ×
×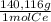 = 0,1468g of Ce
= 0,1468g of Ce 

