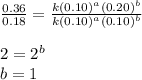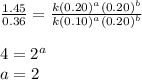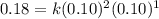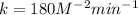The reaction, 2 no(g) + cl2(g) → 2 nocl(g) was studied at -10oc. the following results were obtained. [no] (m) [cl2] (m) initial rate (m min-1) 0.10 0.10 0.18 0.10 0.20 0.36 0.20 0.20 1.45 a) what is the rate law? b) what is the value of the rate constant? (include correct units)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1
You know the right answer?
The reaction, 2 no(g) + cl2(g) → 2 nocl(g) was studied at -10oc. the following results were obtained...
Questions









Social Studies, 26.11.2021 06:00



English, 26.11.2021 06:00

Physics, 26.11.2021 06:00




Mathematics, 26.11.2021 06:00

History, 26.11.2021 06:00


Mathematics, 26.11.2021 06:00

![\text{Rate}=k[NO]^2[Cl_2]^1](/tpl/images/0351/5888/c1bb3.png)
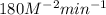
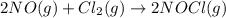
![\text{Rate}=k[NO]^a[Cl_2]^b](/tpl/images/0351/5888/01b4d.png)
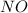
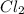
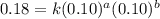 ....(1)
....(1)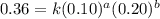 ....(2)
....(2)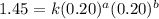 ....(3)
....(3)