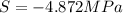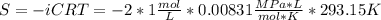Chemistry, 29.10.2019 21:31 tynyiaawrightt
Assuming that the nacl is completely ionized, calculate how much it will lower the solute potential of the soil at 20°c using the solute potential equation: ѱs = –icrt where i is the ionization constant (2 for nacl), c is the molar concentration (in mol/l), r is the pressure constant [r = 0.00831 l • mpa/(mol • k)], and t is the temperature in kelvin (273 + °c). how much will the solute potential of the soil be lowered at 20°c?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
You know the right answer?
Assuming that the nacl is completely ionized, calculate how much it will lower the solute potential...
Questions






Mathematics, 29.09.2021 20:00


Mathematics, 29.09.2021 20:00



Chemistry, 29.09.2021 20:00

English, 29.09.2021 20:00