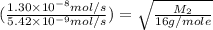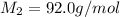Chemistry, 29.10.2019 19:31 Hippiekoolaid
Methane (ch4) effuses through a small opening in the side of a container at the rate of 1.30 × 10 8 mol s 1. an unknown gas effuses through the same opening at the rate of 5.42 × 10 9 mol s 1 when maintained at the same tem- perature and pressure as the methane. determine the molar mass of the unknown gas.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
Methane (ch4) effuses through a small opening in the side of a container at the rate of 1.30 × 10 8...
Questions

Computers and Technology, 04.08.2019 09:00

English, 04.08.2019 09:00

Mathematics, 04.08.2019 09:00


Biology, 04.08.2019 09:00

History, 04.08.2019 09:00



Mathematics, 04.08.2019 09:00

Mathematics, 04.08.2019 09:00




Mathematics, 04.08.2019 09:00



Biology, 04.08.2019 09:00

Spanish, 04.08.2019 09:00


Mathematics, 04.08.2019 09:00

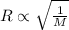
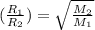 ..........(1)
..........(1)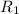 = rate of effusion of methane gas =
= rate of effusion of methane gas = 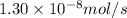
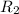 = rate of effusion of unknown gas =
= rate of effusion of unknown gas = 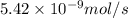
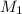 = molar mass of methane gas = 16 g/mole
= molar mass of methane gas = 16 g/mole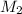 = molar mass of unknown gas = ?
= molar mass of unknown gas = ?