
Chemistry, 29.10.2019 04:31 21ghostrider21
The first step in the process used to recover zinc metal from zinc sulfide ore is the reaction of zinc sulfide with oxygen gas to produce zinc oxide and sulfur dioxide. 2zns(s) 3o2(g)⟶2zno(s) 2so2(g) when the external pressure is 1.523×105 pa and the temperature is 675 k, the amount of work performed is 850.1 j. calculate how many grams of oxygen are consumed in the reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
You know the right answer?
The first step in the process used to recover zinc metal from zinc sulfide ore is the reaction of zi...
Questions

Mathematics, 05.12.2020 04:00

Geography, 05.12.2020 04:00


Biology, 05.12.2020 04:00

Mathematics, 05.12.2020 04:00


Mathematics, 05.12.2020 04:00

Mathematics, 05.12.2020 04:00

Mathematics, 05.12.2020 04:00

Mathematics, 05.12.2020 04:00

Biology, 05.12.2020 04:00





History, 05.12.2020 04:00



Mathematics, 05.12.2020 04:00

History, 05.12.2020 04:00


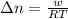
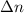 = change in moles of gas = ?
= change in moles of gas = ?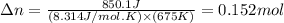
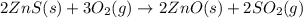
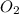 = 32 g/mol
= 32 g/mol

