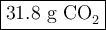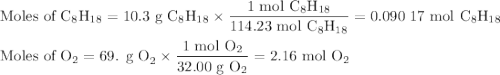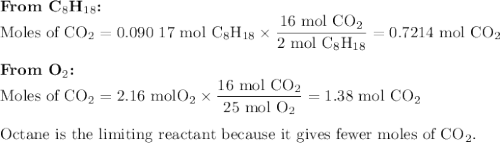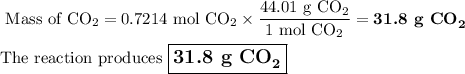Chemistry, 29.10.2019 04:31 iamabouttofail
Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 10.3 g of octane is mixed with 69. g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. round your answer to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1
You know the right answer?
Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . s...
Questions


Mathematics, 18.03.2021 01:10


Mathematics, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10



Mathematics, 18.03.2021 01:10


Computers and Technology, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10

Biology, 18.03.2021 01:10

Spanish, 18.03.2021 01:10



Geography, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10

Chemistry, 18.03.2021 01:10