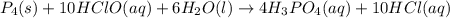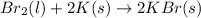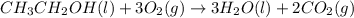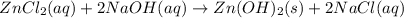p4(s)+10hclo(aq)+6h2o(l)? 4h3po4(aq)+10hcl(aq)

Chemistry, 29.10.2019 02:31 AysiaRamosLee
Which of the following are redox reactions?
p4(s)+10hclo(aq)+6h2o(l)? 4h3po4(aq)+10hcl(aq)
br2(l)+2k(s)? 2kbr(s)
ch3ch2oh(l)+3o2(g)? 3h2o(l)+2co2(g)
zncl2(aq)+2naoh(aq)? zn(oh)2(s)+2nacl(aq)
check all that apply.
part bfor those reactions that are redox, indicate which elements are oxidized. express your answers as chemical symbols separated by a commas. part cfor those reactions that are redox, indicate which elements are reduced. express your answers as chemical symbols separated by a commas. part dfor those reactions that are not redox, indicate whether they are precipitation or neutralization reactions.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1


Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2
You know the right answer?
Which of the following are redox reactions?
p4(s)+10hclo(aq)+6h2o(l)? 4h3po4(aq)+10hcl(aq)
p4(s)+10hclo(aq)+6h2o(l)? 4h3po4(aq)+10hcl(aq)
Questions

Computers and Technology, 26.06.2020 16:01


Computers and Technology, 26.06.2020 16:01


Computers and Technology, 26.06.2020 16:01



Mathematics, 26.06.2020 16:01


Mathematics, 26.06.2020 16:01


Mathematics, 26.06.2020 16:01


Biology, 26.06.2020 16:01