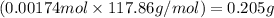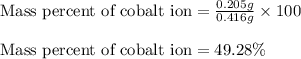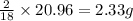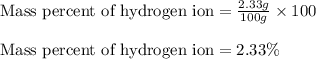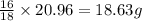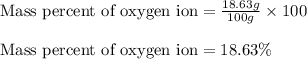In most of its ionic compounds, cobalt is either co(ii) or co(iii). one such compound, containing chloride ion and waters of hydration, was analyzed, and the following results were obtained. a 0.256-g sample of the compound was dissolved in water, and excess silver nitrate was added. the silver chloride was filtered, dried, and weighed, and it had a mass of 0.308 g. a second sample of 0.416 g of the compound was dissolved in water, and an excess of sodium hydroxide was added. the hydroxide salt was filtered and heated in a flame, forming cobalt(iii) oxide. the mass of the cobalt(iii) oxide formed was 0.145 g. what is the percent composition, by mass, of the compound?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
In most of its ionic compounds, cobalt is either co(ii) or co(iii). one such compound, containing ch...
Questions

Social Studies, 30.07.2019 13:20



Social Studies, 30.07.2019 13:20

History, 30.07.2019 13:20

Mathematics, 30.07.2019 13:20


Mathematics, 30.07.2019 13:20

Computers and Technology, 30.07.2019 13:20


Mathematics, 30.07.2019 13:20


English, 30.07.2019 13:20

English, 30.07.2019 13:20

History, 30.07.2019 13:20



Mathematics, 30.07.2019 13:20


Biology, 30.07.2019 13:20

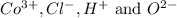 ions in the compound are 49.28 %, 29.79 %, 2.33 % and 18.63 % respectively
ions in the compound are 49.28 %, 29.79 %, 2.33 % and 18.63 % respectively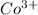 ions = 0.416 grams
ions = 0.416 grams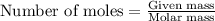 ......(1)
......(1)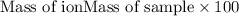 .......(2)
.......(2)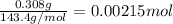
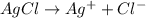
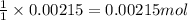 of chloride ions
of chloride ions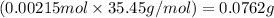
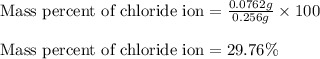
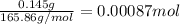
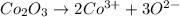
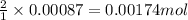 of cobalt ions
of cobalt ions