
Chemistry, 28.10.2019 21:31 altstattlana
Be sure to answer all parts. sulfuric acid is commonly used as an electrolyte in car batteries. suppose you spill some on your garage floor. before cleaning it up, you wisely decide to neutralize it with sodium bicarbonate (baking soda) from your kitchen. the reaction of sodium bicarbonate and sulfuric acid is na2co3(s) + h2so4(aq) → na2so4(aq) + 2co2(g) + 2h2o(l) you estimate that your acid spill contains about 5.1 mol h2so4. what mass of nahco3 do you need to neutralize the acid

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which of the following mining methods disrupts the sea floor?
Answers: 1

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 06:10
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3
You know the right answer?
Be sure to answer all parts. sulfuric acid is commonly used as an electrolyte in car batteries. supp...
Questions

English, 20.09.2021 14:30


Physics, 20.09.2021 14:30

Mathematics, 20.09.2021 14:30


Mathematics, 20.09.2021 14:30

Mathematics, 20.09.2021 14:30

Biology, 20.09.2021 14:30



Mathematics, 20.09.2021 14:30


English, 20.09.2021 14:40

Health, 20.09.2021 14:40




Mathematics, 20.09.2021 14:40

Spanish, 20.09.2021 14:40

Mathematics, 20.09.2021 14:40

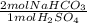 = 10.2 mol NaHCO₃
= 10.2 mol NaHCO₃

