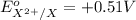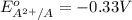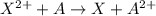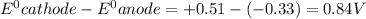Chemistry, 13.10.2019 13:30 chevystewart6628
Atoms a and x are fictional atoms. suppose that the standard potential for the reduction of x^2+ is +0.51 v, and the standard potential for the reduction of a^2+ is -0.33. find the standard potential for an electrochemical cell with the cell reaction that follows.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3
You know the right answer?
Atoms a and x are fictional atoms. suppose that the standard potential for the reduction of x^2+ is...
Questions


Mathematics, 16.10.2019 22:40









Mathematics, 16.10.2019 22:40

Business, 16.10.2019 22:40



Mathematics, 16.10.2019 22:40

Biology, 16.10.2019 22:40

Mathematics, 16.10.2019 22:40



Mathematics, 16.10.2019 22:40

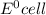 = standard electrode potential =
= standard electrode potential =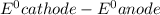
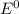 values have to be reduction potentials.
values have to be reduction potentials.