
Chemistry, 27.10.2019 02:43 Queenofpizza
Determine the mass in grams of 3.00 × 10²¹ atoms of arsenic. (the mass of one mole of arsenic is 74.92 g.)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Select the correct answer. what is the nature of the se-cl bond in a molecule of selenium chloride (secl2) if the electronegativity value of selenium is 2.55 and that of chlorine is 3.16?
Answers: 3

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1
You know the right answer?
Determine the mass in grams of 3.00 × 10²¹ atoms of arsenic. (the mass of one mole of arsenic is 74....
Questions



Mathematics, 02.09.2021 17:10

Physics, 02.09.2021 17:10


Biology, 02.09.2021 17:10

Engineering, 02.09.2021 17:10


Mathematics, 02.09.2021 17:10

Biology, 02.09.2021 17:10

History, 02.09.2021 17:10

Mathematics, 02.09.2021 17:10

Advanced Placement (AP), 02.09.2021 17:10

English, 02.09.2021 17:10

Social Studies, 02.09.2021 17:10



Mathematics, 02.09.2021 17:10


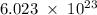 .
.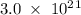 atoms.
atoms.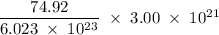 grams
grams

