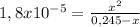Chemistry, 26.10.2019 05:43 blanca04fp
The degree to which a weak base dissociates is given by the base-ionization constant, kb. for the generic weak base, b b(aq)+h2o(l)⇌bh+(aq)+oh−(aq) this constant is given by kb=[bh+][oh−][b] strong bases will have a higher kb value. similarly, strong bases will have a higher percent ionization value. percent ionization=[oh−] equilibrium[b] initial×100% strong bases, for which kb is very large, ionize completely (100%). for weak bases, the percent ionization changes with concentration. the more dilute the solution, the greater the percent ionization. ammonia, nh3, is a weak base with a kb value of 1.8×10−5.parta what is the ph of a 0.245 m ammonia solution? partb what is the percent ionization of ammonia at this concentration?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

You know the right answer?
The degree to which a weak base dissociates is given by the base-ionization constant, kb. for the ge...
Questions





Social Studies, 07.01.2021 20:50

English, 07.01.2021 20:50



Mathematics, 07.01.2021 20:50


History, 07.01.2021 20:50

English, 07.01.2021 20:50

English, 07.01.2021 20:50


Mathematics, 07.01.2021 20:50

Computers and Technology, 07.01.2021 20:50

Mathematics, 07.01.2021 20:50


Health, 07.01.2021 20:50