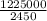Chemistry, 26.10.2019 03:43 Deavionaaaaa
An atom in its ground state is excited when it absorbs a single photon of light. the atom then relaxes back to the ground state by emitting two photons, the first, a red photon at 700 nm, and the second, an infrared photon at 1750 nm. what is the wavelength of the absorbed photon? 500 nm 1225 nm 700 nm 1950 nm 1750 nm

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1
You know the right answer?
An atom in its ground state is excited when it absorbs a single photon of light. the atom then relax...
Questions

Mathematics, 01.06.2021 20:20



Social Studies, 01.06.2021 20:20


Biology, 01.06.2021 20:20

English, 01.06.2021 20:20


History, 01.06.2021 20:20



Social Studies, 01.06.2021 20:20

Mathematics, 01.06.2021 20:20




History, 01.06.2021 20:20



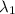 ) = 700 nm
) = 700 nm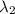 ) = 1750 nm
) = 1750 nm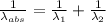
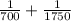
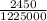
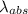 =
=