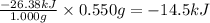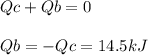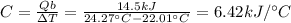Chemistry, 26.10.2019 02:43 holman9308
The combustion of exactly 1.000 g of benzoic acid in a bomb calorimeter releases 26.38 kj of heat. if the combustion of 0.550 g of benzoic acid causes the temperature of the calorimeter to increase from 22.01∘c to 24.27∘c, calculate the heat capacity of the calorimeter.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 23.06.2019 07:00
In order for a high temperature boiler or steam engine to produce superheated water, or steam: the heat source must be greater than 100°c the water must be permitted to evaporate quickly the system must be sealed and become pressurized above atmospheric pressure the vapor pressure must be kept below 760 mm(hg)
Answers: 1
You know the right answer?
The combustion of exactly 1.000 g of benzoic acid in a bomb calorimeter releases 26.38 kj of heat. i...
Questions

English, 10.01.2021 01:10

Mathematics, 10.01.2021 01:10


History, 10.01.2021 01:10


Mathematics, 10.01.2021 01:10


Spanish, 10.01.2021 01:10

History, 10.01.2021 01:10

Mathematics, 10.01.2021 01:10

Mathematics, 10.01.2021 01:10


Chemistry, 10.01.2021 01:10




Mathematics, 10.01.2021 01:10

Mathematics, 10.01.2021 01:10


Mathematics, 10.01.2021 01:10