
Chemistry, 26.10.2019 00:43 gizmo50245
Aspirin (c9h8o4) is synthesized by reacting salicylic acid (c7h6o3) with acetic anhydride (c4h6o3) according to the reaction: c7h6o3 + c4h6o3 --> c9h8o4 + hc2h3o2 a. what mass of acetic anhydride is needed to completely react 1.00 x 10 4 g of salicylic acid?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

You know the right answer?
Aspirin (c9h8o4) is synthesized by reacting salicylic acid (c7h6o3) with acetic anhydride (c4h6o3) a...
Questions

Chemistry, 30.09.2019 13:00



History, 30.09.2019 13:00



Mathematics, 30.09.2019 13:00


Mathematics, 30.09.2019 13:00



Biology, 30.09.2019 13:00




Biology, 30.09.2019 13:00




Mathematics, 30.09.2019 13:00

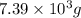 of acetic anhydride is needed
of acetic anhydride is needed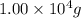 of salicylic acid =
of salicylic acid = 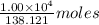 of salicylic acid
of salicylic acid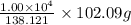 =
= 

