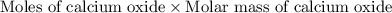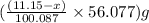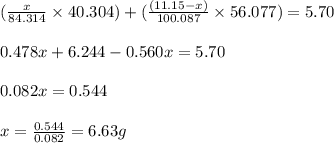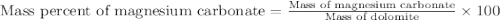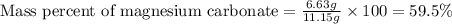Chemistry, 26.10.2019 00:43 mckadams02
Dolomite is a mixed carbonate of calcium and magnesium that decomposes to co2 and the metal oxides mgo and cao upon heating. when 11.15 g of dolomite is heated, 5.70 g of mgo and cao are produced. what is percent by mass of mgco3 in the original sample of dolomite?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1


Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
Dolomite is a mixed carbonate of calcium and magnesium that decomposes to co2 and the metal oxides m...
Questions



Chemistry, 08.07.2019 02:30

Mathematics, 08.07.2019 02:30

Mathematics, 08.07.2019 02:30



Mathematics, 08.07.2019 02:30

Mathematics, 08.07.2019 02:30



English, 08.07.2019 02:30

Mathematics, 08.07.2019 02:30

English, 08.07.2019 02:30


Mathematics, 08.07.2019 02:30

Mathematics, 08.07.2019 02:30

Computers and Technology, 08.07.2019 02:30

Geography, 08.07.2019 02:30

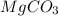 in dolomite is 59.5 %
in dolomite is 59.5 %
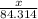 moles
moles 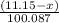 moles
moles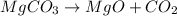
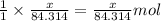 of magnesium oxide
of magnesium oxide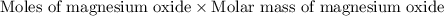
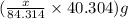

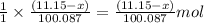 of calcium oxide
of calcium oxide