
Chemistry, 25.10.2019 23:43 saabrrinnaaa
At 650 k, the reaction mgco3(s)⇌mgo(s)+co2(g)mgco3(s)⇌mgo( s)+co2(g) has kp=0.026kp=0.026. a 10.0-l container at 650 k has 1.0 g of mgo(s) and co2co2 at p=0.0260 atmp=0.0260 atm. the container is then compressed to a volume of 0.100 l. find the mass of mgco3mgco3 that is formed.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:10
What can be added to the examples section of each circle? endothermic: ice melting into water, and a heat pack becoming warm exothermic: a glow stick glowing, and fireworks exploding endothermic: ice melting into water, and an instant ice pack turning cold exothermic: fireworks exploding, and gasoline burning endothermic: a glow stick glowing, and a heat pack becoming warm exothermic: an instant ice pack turning cold, and ice melting into water endothermic: gasoline burning, and an instant ice pack turning cold exothermic: ice melting into water, and an instant ice pack turning cold
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3


You know the right answer?
At 650 k, the reaction mgco3(s)⇌mgo(s)+co2(g)mgco3(s)⇌mgo( s)+co2(g) has kp=0.026kp=0.026. a 10.0-l...
Questions

Mathematics, 26.07.2021 01:00

Mathematics, 26.07.2021 01:00


Mathematics, 26.07.2021 01:00


Social Studies, 26.07.2021 01:00

English, 26.07.2021 01:00


Advanced Placement (AP), 26.07.2021 01:10


Mathematics, 26.07.2021 01:10

Chemistry, 26.07.2021 01:10



Mathematics, 26.07.2021 01:10


History, 26.07.2021 01:10


History, 26.07.2021 01:10

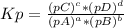 , where pX is the pressure of X in equilibrium.
, where pX is the pressure of X in equilibrium. 

