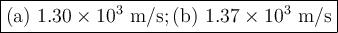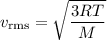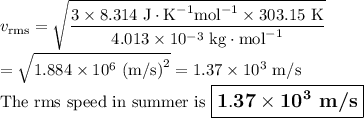Helium (he) is the lightest noble gas component of air, and xenon (xe) is the heaviest. perform the following calculations, using r = 8.314 j/(mol·k) and ℳ in kg/mol. (a) find the rms speed of he in winter (0.°c) and in summer (30.°c). enter your answers in scientific notation.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2
You know the right answer?
Helium (he) is the lightest noble gas component of air, and xenon (xe) is the heaviest. perform the...
Questions


Mathematics, 26.10.2020 20:00


Physics, 26.10.2020 20:00


Social Studies, 26.10.2020 20:00

English, 26.10.2020 20:00

History, 26.10.2020 20:00

Mathematics, 26.10.2020 20:00


Mathematics, 26.10.2020 20:00


Mathematics, 26.10.2020 20:00

Mathematics, 26.10.2020 20:00



Physics, 26.10.2020 20:00


Chemistry, 26.10.2020 20:00

History, 26.10.2020 20:00