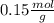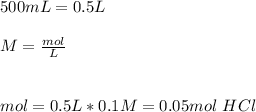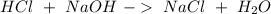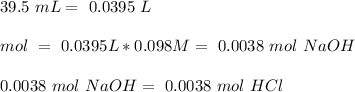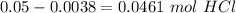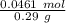Chemistry, 25.10.2019 20:43 alexisolermeador389
Astudent used 0.29 g of a sample and added to it 50.0 ml of 0.100 m hcl. the resulting solution then required 39.50 ml of 0.0980 m naoh to reach a ph of 3.0. what is the acid consuming capacity of the sample in moles of acid neutralized per gram of sample?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1
You know the right answer?
Astudent used 0.29 g of a sample and added to it 50.0 ml of 0.100 m hcl. the resulting solution then...
Questions

Chemistry, 20.11.2020 18:00

Mathematics, 20.11.2020 18:00

Mathematics, 20.11.2020 18:00




Spanish, 20.11.2020 18:00


Mathematics, 20.11.2020 18:00

English, 20.11.2020 18:00



Mathematics, 20.11.2020 18:00


Mathematics, 20.11.2020 18:00

Mathematics, 20.11.2020 18:00

Mathematics, 20.11.2020 18:00



Mathematics, 20.11.2020 18:00