
Chemistry, 25.10.2019 18:43 jaydenforrest4367
Problem page gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 5.2 g of butane is mixed with 32.6 g of oxygen. calculate the minimum mass of butane that could be left over by the chemical reaction. round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

You know the right answer?
Problem page gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gas...
Questions

Mathematics, 21.08.2019 03:10


Social Studies, 21.08.2019 03:10

Social Studies, 21.08.2019 03:10







Computers and Technology, 21.08.2019 03:10





Geography, 21.08.2019 03:10




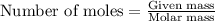
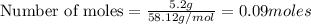
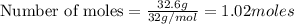
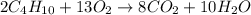
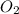
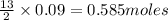 of
of 

