Determine the ph of a 0.500 m hno2 solution. ka of hno2 is 4.6 * 10-4.
a. 1.82
b....


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 23.06.2019 05:30
Find the midpoint of a segment with endpoints of 4-3i and -2+7i
Answers: 2

Chemistry, 23.06.2019 07:00
If you used the method of initial rates to obtain the order for no2, predict what reaction rates you would measure in the beginning of the reaction for initial concentrations of 0.200 m, 0.100 m, & 0.050 m no2.
Answers: 3

Chemistry, 23.06.2019 09:30
If the solubility of a gas in water is 1.22g/2.75 atm, what is it’s solubility (in g/l) at 1.0 atm
Answers: 1
You know the right answer?
Questions



Mathematics, 25.10.2020 22:40

Mathematics, 25.10.2020 22:40


Physics, 25.10.2020 22:40

Mathematics, 25.10.2020 22:40

Spanish, 25.10.2020 22:40

Mathematics, 25.10.2020 22:40




Geography, 25.10.2020 22:40

Social Studies, 25.10.2020 22:40


Mathematics, 25.10.2020 22:40

Mathematics, 25.10.2020 22:40



Mathematics, 25.10.2020 22:40

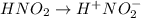
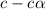
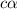
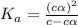
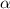 = ?
= ?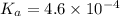
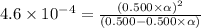
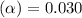
![[H^+]=c\times \alpha](/tpl/images/0346/2002/4fc41.png)
![[H^+]=0.500\times 0.030=0.015](/tpl/images/0346/2002/28636.png)
![pH=-log[H^+]](/tpl/images/0346/2002/15713.png)
![pH=-log[0.015]=1.82](/tpl/images/0346/2002/45771.png)
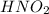 solution is 1.82
solution is 1.82

