
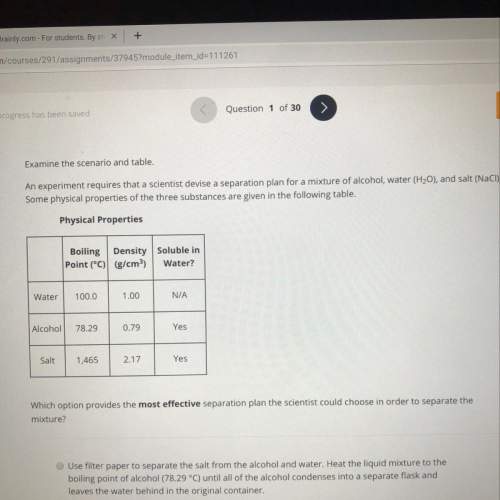
A. use filter paper to separate the salt from the alcohol and water. heat the liquid mixture to the
boiling point of alcohol (78.29 °c) until all of the alcohol condenses into a separate flask and
leaves the water behind in the original container.
b. heat the mixture to the boiling point of alcohol (78.29 °c), allowing the alcohol to condense into a
separate flask. continue to heat the liquid to a temperature of 100 °c to distill off the water. the
salt will be left behind in the flask.
c. heat the mixture to the boiling point of water (100 °c), allowing the water to condense into a
separate flask. continue to heat the liquid to a temperature of 78.29 °c to distill off the alcohol.
the salt will be left behind in the flask.
d. use filter paper to separate the salt from the alcohol and water. heat the liquid mixture to the
boiling point of alcohol (78.29 °c) and allow the alcohol to condense into a second container.
continue to heat the mixture to a temperature of 100 °c to distill off the water.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1


You know the right answer?
A. use filter paper to separate the salt from the alcohol and water. heat the liquid mixture to the<...
Questions






Mathematics, 21.08.2020 01:01



English, 21.08.2020 01:01

Mathematics, 21.08.2020 01:01

Computers and Technology, 21.08.2020 01:01


History, 21.08.2020 01:01


Mathematics, 21.08.2020 01:01





Biology, 21.08.2020 01:01



