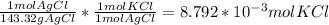Chemistry, 25.10.2019 00:43 cookiebrain72
Consider the following balanced chemical equation, kcl(aq) agno3(aq) → agcl(s) kno3(aq)when a sample of impure potassium chloride (0.900 g) was dissolved in water, and treated with excess silver nitrate (agno3), 1.26 g of silver chloride (agcl) was precipitated. calculate the percentage kcl in the original sample.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3
You know the right answer?
Consider the following balanced chemical equation, kcl(aq) agno3(aq) → agcl(s) kno3(aq)when a sample...
Questions


Business, 03.05.2020 13:37


Social Studies, 03.05.2020 13:37




Mathematics, 03.05.2020 13:37

Biology, 03.05.2020 13:37

Mathematics, 03.05.2020 13:37






Mathematics, 03.05.2020 13:37

English, 03.05.2020 13:37


Mathematics, 03.05.2020 13:37