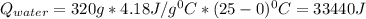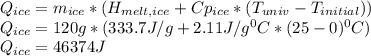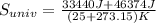Chemistry, 24.10.2019 17:43 chris858232
In a vacuum bottle, 320 g of water and 120 g of ice are initially in equilibrium at 0.00∘∘c. the bottle is not a perfect insulator. over time, its contents come to thermal equilibrium with the outside air at 25.0∘∘c. 1) how much does the entropy of universe increase in the process?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1

Chemistry, 23.06.2019 14:00
How can a ringing telephone can be heard through a closed door
Answers: 1

Chemistry, 23.06.2019 17:00
Using this reversible reaction, answer the questions below: n2o4 2no2 (colorless) (reddish-brown) -as the temperature increased, what happened to the n2o4 concentration? -was the formation of reactants or products favored by the addition of heat? -which reaction is exothermic? right to left or left to right? -if the change of enthalpy of this reaction when proceeding left to right is 14 kcal, which chemical equation is correct? n2o4 2no2 + 14 kcal n2o4 2no2, hr = +14 kcal n2o4 + 14 kcal 2no2 n2o4 2no2, hr = -14 kcal
Answers: 1
You know the right answer?
In a vacuum bottle, 320 g of water and 120 g of ice are initially in equilibrium at 0.00∘∘c. the bot...
Questions

Mathematics, 03.03.2021 21:20

Mathematics, 03.03.2021 21:20


Social Studies, 03.03.2021 21:20

Spanish, 03.03.2021 21:20

Mathematics, 03.03.2021 21:20

Mathematics, 03.03.2021 21:20

Biology, 03.03.2021 21:20



Mathematics, 03.03.2021 21:20

Mathematics, 03.03.2021 21:20



Mathematics, 03.03.2021 21:20

Health, 03.03.2021 21:20

Mathematics, 03.03.2021 21:20


Mathematics, 03.03.2021 21:20

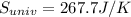
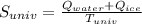
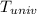 equals the outside air temperature. Then, we must compute both the water's and ice's released heat due to their interaction with the "hot" air:
equals the outside air temperature. Then, we must compute both the water's and ice's released heat due to their interaction with the "hot" air: