
Chemistry, 23.10.2019 23:00 robbinsjeffrey271
Asample of nitrosyl bromide (nobr) decomposes according to the equation 2nobr(g)⇌2no(g)+br2(g) an equilibrium mixture in a 5.00-l vessel at 100 ∘c contains 3.29 g of nobr, 3.04 g of no, and 8.10 g of br2. part apart complete calculate kc. express your answer using three significant figures. 0.116 previous answers correct part bpart complete what is the total pressure exerted by the mixture of gases? express your answer to three significant figures and include the appropriate units. 1.11 atm previous answers correct part c what was the mass of the original sample of nobr?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
There is an area in idaho named craters of the moon where most of the ground is covered with basalt, adark gray, igneous rock with no visibl crystals. what can you infer about the geographical history of the area?
Answers: 1

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3
You know the right answer?
Asample of nitrosyl bromide (nobr) decomposes according to the equation 2nobr(g)⇌2no(g)+br2(g) an eq...
Questions

Mathematics, 04.08.2020 14:01







Mathematics, 04.08.2020 14:01



Mathematics, 04.08.2020 14:01

Mathematics, 04.08.2020 14:01



Chemistry, 04.08.2020 14:01

Computers and Technology, 04.08.2020 14:01

Computers and Technology, 04.08.2020 14:01



![kc = \frac{[Br_{2}][NO]^2{[NOBr]^2}](/tpl/images/0343/4270/3b92b.png) (1)
(1)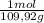 = 0,0299 moles/ 5,00L = 5,986x10⁻³M
= 0,0299 moles/ 5,00L = 5,986x10⁻³M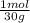 = 0,101 moles/ 5,00L = 0,0203M
= 0,101 moles/ 5,00L = 0,0203M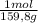 = 0,0507 moles/ 5,00L = 0,0101M
= 0,0507 moles/ 5,00L = 0,0101M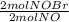 = 0,101 mol NOBr
= 0,101 mol NOBr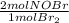 = 0,101 mol NOBr
= 0,101 mol NOBr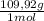 = 25,5 g of NOBr
= 25,5 g of NOBr

