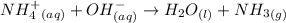Chemistry, 23.10.2019 23:00 Hailey1313131313
2hclo4(aq)+na2co3(aq)→h2o(l)+co2(g) +2naclo4(aq) express your answer as a complete ionic equation. identify all of the phases in your answer. nothing request answer part f express your answer as a net ionic equation. identify all of the phases in your answer. nothing request answer part g nh4cl(aq)+naoh(aq)→h2o(l)+nh3(g)+na cl(aq) express your answer as a complete ionic equation. identify all of the phases in your answer. nothing request answer part h express your answer as a net ionic equation. identify all of the phases in your answer. nothing request answer provide feedback correct. no additional followup.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2
You know the right answer?
2hclo4(aq)+na2co3(aq)→h2o(l)+co2(g) +2naclo4(aq) express your answer as a complete ionic equation. i...
Questions


Mathematics, 17.03.2021 23:40








Social Studies, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40


Biology, 17.03.2021 23:40



History, 17.03.2021 23:40




History, 17.03.2021 23:40

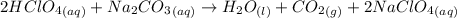
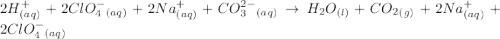
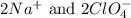 are the spectator ions.
are the spectator ions.
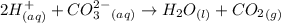
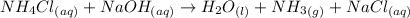
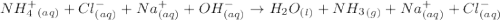
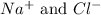 are the spectator ions.
are the spectator ions.