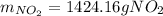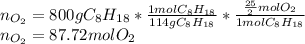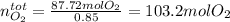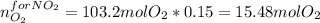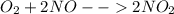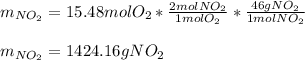The production of nox gases is an unwanted side reaction of the main engine combustion process that turns octane, c8h18, into co2 and water. if 85% of the oxygen in an engine is used to combust octane, and the remainder used to produce nitrogen dioxide, calculate how many grams of nitrogen dioxide would be produced during the combustion of 800 grams of octane.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
The production of nox gases is an unwanted side reaction of the main engine combustion process that...
Questions




Mathematics, 28.07.2019 23:00

Geography, 28.07.2019 23:00


History, 28.07.2019 23:00

History, 28.07.2019 23:00


Mathematics, 28.07.2019 23:00




Mathematics, 28.07.2019 23:00

Biology, 28.07.2019 23:00



History, 28.07.2019 23:00

Mathematics, 28.07.2019 23:00