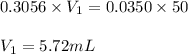Chemistry, 23.10.2019 22:30 Bryanguzman2004
You wish to prepare 50.00 ml of 0.0350 m nh3 from concentrated ammonia (15.28 m) by serial dilution using a 50 ml volumetric flasks, a 1.00 ml volumetric pipet, and a 10.00 ml graduated pipet. for the first dilution in the series, 1 ml of concentrated ammonia is added to a 50.00 volumetric flask and then filled to the line. the solution is mixed thoroughly, poured into a beaker, and then used to make the second solution in the series. what volume of the first solution should be used to make the second solution, 50.00 ml of 0.0350 m nh3 solution, using the glassware provided?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:30
One of the reactions in a blast furnace used to reduce iron is shown above. how many grams of fe2o3 are required to produce 15.5 g of fe if the reaction occurs in the presence of excess co? a.11.1 g b.22.1 g c.30.0 g d.44.2 g
Answers: 2

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
You know the right answer?
You wish to prepare 50.00 ml of 0.0350 m nh3 from concentrated ammonia (15.28 m) by serial dilution...
Questions


English, 17.11.2020 17:40

Chemistry, 17.11.2020 17:40

English, 17.11.2020 17:40

Mathematics, 17.11.2020 17:40

Mathematics, 17.11.2020 17:40


Mathematics, 17.11.2020 17:40




Biology, 17.11.2020 17:40



Mathematics, 17.11.2020 17:40

History, 17.11.2020 17:40

Mathematics, 17.11.2020 17:40



Mathematics, 17.11.2020 17:40

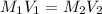 .......(1)
.......(1)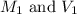 are the molarity and volume of the concentrated ammonia solution
are the molarity and volume of the concentrated ammonia solution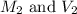 are the molarity and volume of diluted ammonia solution
are the molarity and volume of diluted ammonia solution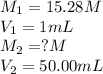
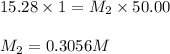
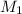 = Molarity of ammonia solution from first dilution = 0.3056 M
= Molarity of ammonia solution from first dilution = 0.3056 M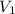 = Volume of ammonia solution from first dilution = ?
= Volume of ammonia solution from first dilution = ?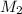 = Molarity of ammonia to make second solution = 0.0350 M
= Molarity of ammonia to make second solution = 0.0350 M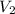 = Volume of ammonia to make second solution = 50.00 mL
= Volume of ammonia to make second solution = 50.00 mL