
Chemistry, 23.10.2019 20:00 hetdashadia123
Ethanol, c2h5oh, is produced industrially from ethylene, c2h4, by the following sequence of reactions: 3c2h4 + 2h2so4 → c2h5hso4 + (c2h5)2so4 c2h5hso4 + (c2h5)2so4 + 3h2o → 3c2h5oh + 2h2so4 what volume of ethylene at stp is required to produce 1.000 metric ton (1000 kg) of ethanol if the overall yield of ethanol is 90.1%

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
Ethanol, c2h5oh, is produced industrially from ethylene, c2h4, by the following sequence of reaction...
Questions


Mathematics, 24.08.2021 03:00

Mathematics, 24.08.2021 03:00

Mathematics, 24.08.2021 03:00


Mathematics, 24.08.2021 03:00


Mathematics, 24.08.2021 03:00

Mathematics, 24.08.2021 03:00

Mathematics, 24.08.2021 03:00

Mathematics, 24.08.2021 03:00






Social Studies, 24.08.2021 03:00



Spanish, 24.08.2021 03:00

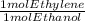 * 100/90.1 = 2.4128* 10⁴ mol ethylene
* 100/90.1 = 2.4128* 10⁴ mol ethylene


