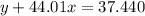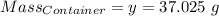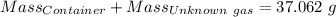Chemistry, 23.10.2019 20:30 camirialchambers17
Agas cylinder filled with nitrogen at standard temperature and pressure has a mass of 37.289 g. the same container filled with carbon dioxide at stp has a mass of 37.440 g. when filled with an unknown gas at stp, the container mass is 37.062 g. calculate the molecular weight of the unknown gas, and then state its probable identity.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 23.06.2019 09:00
Chortling is used to clean water. another possible atom that would also work is a. sodium b. sulfur c. bromine
Answers: 1

Chemistry, 23.06.2019 09:00
What sources of error may have contributed to the percent yield not being 100 percent? think about things that may have led to inaccurate measurements or where mass of the product could have been lost if this experiment was conducted in a physical laboratory.
Answers: 2
You know the right answer?
Agas cylinder filled with nitrogen at standard temperature and pressure has a mass of 37.289 g. the...
Questions

Health, 09.08.2021 05:50






English, 09.08.2021 05:50




Mathematics, 09.08.2021 05:50





Arts, 09.08.2021 06:00

English, 09.08.2021 06:00

Mathematics, 09.08.2021 06:00

Mathematics, 09.08.2021 06:00


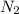 = 28.014 g/mol
= 28.014 g/mol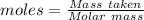
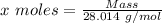
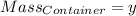
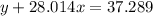
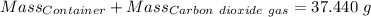
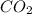 = 44.01 g/mol
= 44.01 g/mol