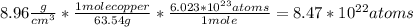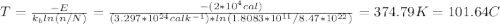Calculate the concentration of vacancies in copper at room temperature (25oc). what temperature will be needed to heat treat copper such that the concentration of vacancies produced will be 1000 times more than the equilibrium concentration of vacancies at room temperature? assume that 20,000 cal are required to produce a mole of vacancies in copper.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1


Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1
You know the right answer?
Calculate the concentration of vacancies in copper at room temperature (25oc). what temperature will...
Questions

Chemistry, 25.04.2021 14:00

Mathematics, 25.04.2021 14:00


Mathematics, 25.04.2021 14:00

Mathematics, 25.04.2021 14:00

Mathematics, 25.04.2021 14:00

Mathematics, 25.04.2021 14:00

English, 25.04.2021 14:00

History, 25.04.2021 14:00


Spanish, 25.04.2021 14:00



Biology, 25.04.2021 14:00

Business, 25.04.2021 14:00

Business, 25.04.2021 14:00

Advanced Placement (AP), 25.04.2021 14:00

Mathematics, 25.04.2021 14:00

English, 25.04.2021 14:00