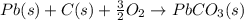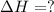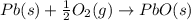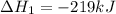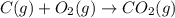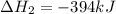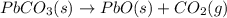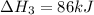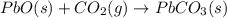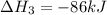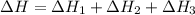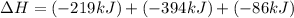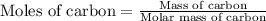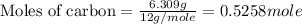Chemistry, 23.10.2019 19:30 desiiraee6265
Use the following information to calculate the amount of heat involved in the complete reaction of 6.309 of carbon to from pbco3 (s) in reaction 4. be sure to give the proper sign (positive or negative) with your answer. (1) pb(s)+1/2 o2 right arrow pbo(s) (2) c(g)+o2(g) right arrow co2(g) (3) pbco3(s)right arrow pbo(s)+co2(g) (4) pb(s)+c(s)+3/2 o2(g) right arrow pbo3(s) delta h degree rsn= -219 kj delta h degree rsn= -394 kj delta h degree rsn= 86 kj

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
Use the following information to calculate the amount of heat involved in the complete reaction of 6...
Questions



Mathematics, 06.04.2020 15:22




Mathematics, 06.04.2020 15:23


Mathematics, 06.04.2020 15:23






Mathematics, 06.04.2020 15:24




Mathematics, 06.04.2020 15:24

Mathematics, 06.04.2020 15:24