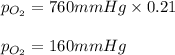Chemistry, 23.10.2019 18:30 dannyelleparker9680
Atmospheric pressure at sea level is equal to a column of 760 mm hg. oxygen makes up 21 percent of the atmosphere by volume. the partial pressure of oxygen (po2) in such conditions is atmospheric pressure at sea level is equal to a column of 760 mm hg. oxygen makes up 21 percent of the atmosphere by volume. the partial pressure of oxygen (po2) in such conditions is 21/760 16 mm hg 760/21 120/75 160 mm hg submitr

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1
You know the right answer?
Atmospheric pressure at sea level is equal to a column of 760 mm hg. oxygen makes up 21 percent of t...
Questions

Mathematics, 05.02.2020 09:44


Geography, 05.02.2020 09:44

Social Studies, 05.02.2020 09:44

Geography, 05.02.2020 09:44



Biology, 05.02.2020 09:44

Social Studies, 05.02.2020 09:44


History, 05.02.2020 09:44

Social Studies, 05.02.2020 09:44



Mathematics, 05.02.2020 09:44

Mathematics, 05.02.2020 09:44

Biology, 05.02.2020 09:44


Mathematics, 05.02.2020 09:44

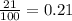
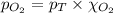
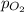 = partial pressure of oxygen = ?
= partial pressure of oxygen = ?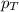 = total pressure of air = 760 mmHg
= total pressure of air = 760 mmHg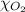 = mole fraction of oxygen = 0.21
= mole fraction of oxygen = 0.21