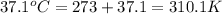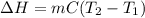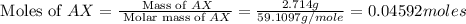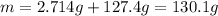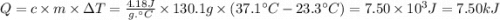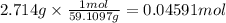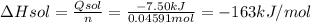Chemistry, 23.10.2019 17:00 mrgandollins5222
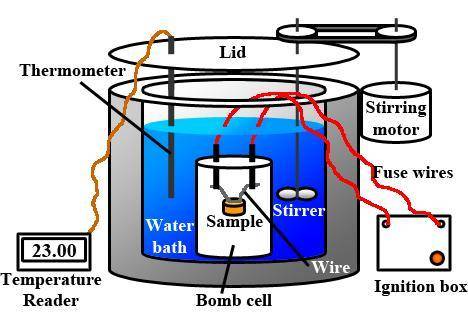
When 2.714 g of ax (s) dissolves in 127.4 g of water in a coffee-cup calorimeter the temperature rises from 23.3 °c to 37.1 °c. calculate the enthalpy change (in kj/mol) for the solution process. ax(s) → a+(aq) + x-(aq) assumptions for this calculation: the specific heat of the solution is the same as that of pure water (4.18 j/gk) the density of water = 1.000 g/ml the liquid’s final volume is not changed by adding the solid the calorimeter loses only a negligible quantity of heat. the formula weight of ax = 59.1097 g/mol. be sure you include the correct sign for the enthalpy change.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Describe in detail the melting point behavior of the 80: 20 benzoic acid-mandelic acid mixture
Answers: 3

Chemistry, 21.06.2019 22:30
Which supports the idea that birds and butterflies both have wings but they do not have a common ancestor with wings? a. the wings are analogous structures that evolved differently and do not have a similar internal structure. b. the wings are homologous structures that evolved differently and do not have a similar internal structure. c. wings of birds are vestigial structures, but the wing structures of bats are not vestigial. d. wings of bats are vestigial structures, but the wing structures of birds are not vestigial
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3
You know the right answer?
When 2.714 g of ax (s) dissolves in 127.4 g of water in a coffee-cup calorimeter the temperature ris...
Questions


Mathematics, 07.10.2019 21:30

Computers and Technology, 07.10.2019 21:30


Geography, 07.10.2019 21:30



Mathematics, 07.10.2019 21:30





Social Studies, 07.10.2019 21:30

Mathematics, 07.10.2019 21:30




Chemistry, 07.10.2019 21:30

Social Studies, 07.10.2019 21:30

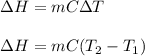
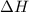 = change in enthalpy = ?
= change in enthalpy = ?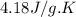
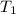 = initial temperature =
= initial temperature = 
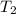 = final temperature =
= final temperature =