
Chemistry, 16.09.2019 05:50 Bleejones00
Write a balanced chemical equation for the reaction of zncl2 with excess naoh to produce na2zn(oh)4 sodium zincate. what mass of sodium zincate can be produced from 2.00 g of zncl2 with excess naoh by this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1
You know the right answer?
Write a balanced chemical equation for the reaction of zncl2 with excess naoh to produce na2zn(oh)4...
Questions

Biology, 09.04.2021 05:10




Biology, 09.04.2021 05:10

Mathematics, 09.04.2021 05:10

Mathematics, 09.04.2021 05:10

Mathematics, 09.04.2021 05:10


Mathematics, 09.04.2021 05:10






Mathematics, 09.04.2021 05:10

Chemistry, 09.04.2021 05:10


Mathematics, 09.04.2021 05:10

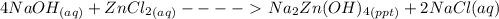
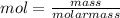
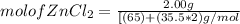
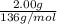
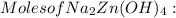
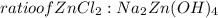
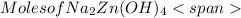 = 0.0147 mol
= 0.0147 mol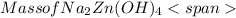 = [(23 * 2)+(65)+(16 * 4) + (1*4)] g/mol * 0.0147 mol
= [(23 * 2)+(65)+(16 * 4) + (1*4)] g/mol * 0.0147 mol

