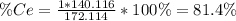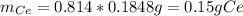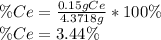Chemistry, 23.10.2019 16:50 chelseychew32
To find the ce4+ content in a solid sample, 4.3718 g of the solid sample were dissolved and treated with excess iodate to precipitate the ce4+ as ce(io3)4. the precipitate was collected, washed well, dried, and ignited to produce 0.1848 g of ceo2 (fm 172.114). what was the weight percentage of ce (am 140.116) in the original sample?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1

You know the right answer?
To find the ce4+ content in a solid sample, 4.3718 g of the solid sample were dissolved and treated...
Questions




Geography, 25.09.2019 05:20








Computers and Technology, 25.09.2019 05:20

History, 25.09.2019 05:20

Mathematics, 25.09.2019 05:20

Computers and Technology, 25.09.2019 05:20




Computers and Technology, 25.09.2019 05:20

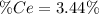
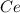 into the
into the 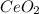 by using their respective molar masses as shown below:
by using their respective molar masses as shown below: