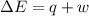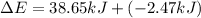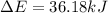Chemistry, 23.10.2019 00:30 kandigirl9990
Limestone stalactites and stalagmites are formed in caves by the following reaction: ca2 (aq) 2hco−3(aq)→caco3(s) co2(g) h2o(l) if 1 mol of caco3 forms at 298 k under 1 atm pressure, the reaction performs 2.47 kj of p−v work, pushing back the atmosphere as the gaseous co2 forms. at the same time, 38.65 kj of heat is absorbed from the environment. what is the value of δe for this reaction

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3
You know the right answer?
Limestone stalactites and stalagmites are formed in caves by the following reaction: ca2 (aq) 2hco−...
Questions


History, 04.08.2019 16:50


History, 04.08.2019 16:50

Biology, 04.08.2019 16:50

Business, 04.08.2019 16:50











Social Studies, 04.08.2019 16:50


Biology, 04.08.2019 16:50

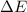 for this reaction is 36.18 kJ
for this reaction is 36.18 kJ