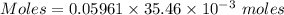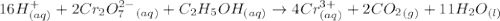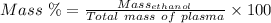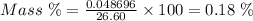Aperson's blood alcohol (c2h5oh) level can be determined by titrating a sample of blood plasma with a potassium dichromate solution. the balanced equation is 16h (aq) 2cr2o72−(aq) c2h5oh(aq) → 4cr3 (aq) 2co2(g) 11h2o(l) if 35.46 ml of 0.05961 m cr2o72− is required to titrate 26.60 g of plasma, what is the mass percent of alcohol in the blood?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1
You know the right answer?
Aperson's blood alcohol (c2h5oh) level can be determined by titrating a sample of blood plasma with...
Questions










English, 18.01.2020 05:31








Mathematics, 18.01.2020 05:31

French, 18.01.2020 05:31

Mathematics, 18.01.2020 05:31

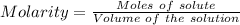
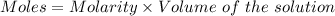
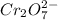 :
: