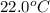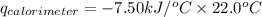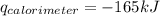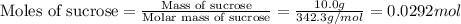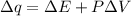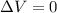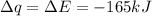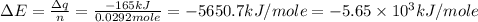Consider the reaction c12h22o11(s)+12o2(g)→12co2(g)+11h2o (l) in which 10.0 g of sucrose, c12h22o11, was burned in a bomb calorimeter with a heat capacity of 7.50 kj/∘c. the temperature increase inside the calorimeter was found to be 22.0 ∘c. calculate the change in internal energy, δe, for this reaction per mole of sucrose. express the change in internal energy in kilojoules per mole to three significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 23.06.2019 07:50
What is the significance sodium hydroxide and hydrochloric acid
Answers: 1

You know the right answer?
Consider the reaction c12h22o11(s)+12o2(g)→12co2(g)+11h2o (l) in which 10.0 g of sucrose, c12h22o11,...
Questions


Business, 12.02.2020 06:40

Mathematics, 12.02.2020 06:40


Mathematics, 12.02.2020 06:40

Mathematics, 12.02.2020 06:40



English, 12.02.2020 06:41

Mathematics, 12.02.2020 06:41



Mathematics, 12.02.2020 06:41

English, 12.02.2020 06:41

Mathematics, 12.02.2020 06:41

Mathematics, 12.02.2020 06:41

History, 12.02.2020 06:41


Arts, 12.02.2020 06:41

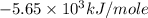
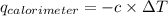
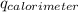 = heat released by calorimeter = ?
= heat released by calorimeter = ?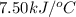
 = change in temperature of calorimeter =
= change in temperature of calorimeter =