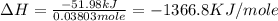Chemistry, 22.10.2019 23:30 hanacat6174
Find δh for the combustion of ethanol (c2h6o) to carbon dioxide and liquid water from the following data. the heat capacity of the bomb calorimeter is 34.65 kj/k and the combustion of 1.752 g of ethanol raises the temperature of the calorimeter from 294.42 k to 295.92 k .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3


Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

You know the right answer?
Find δh for the combustion of ethanol (c2h6o) to carbon dioxide and liquid water from the following...
Questions

Chemistry, 02.10.2019 15:00

History, 02.10.2019 15:00



Mathematics, 02.10.2019 15:00




Social Studies, 02.10.2019 15:00


History, 02.10.2019 15:00




Biology, 02.10.2019 15:00

History, 02.10.2019 15:00


Mathematics, 02.10.2019 15:00

Social Studies, 02.10.2019 15:00

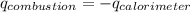
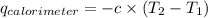
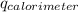 = heat released by calorimeter = ?
= heat released by calorimeter = ?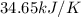
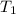 = initial temperature of calorimeter = 294.42 K
= initial temperature of calorimeter = 294.42 K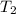 = final temperature of calorimeter = 295.92 K
= final temperature of calorimeter = 295.92 K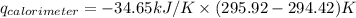
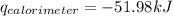
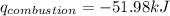
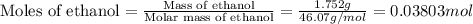
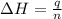
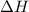 = enthalpy of combustion = ?
= enthalpy of combustion = ?