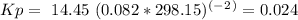Enter your answer in the provided box. a united nations toxicologist studying the properties of mustard gas, s(ch2ch2cl)2, a blistering agent used in warfare, prepares a mixture of 0.675 m scl2and 0.973 m c2h4and allows it to react at room temperature (20.0°c): scl2(g) + 2 c2h4(g) ⇌ s(ch2ch2cl)2(g) at equilibrium,[s( ch2ch2cl)2] = 0.350 m. calculate .

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2
You know the right answer?
Enter your answer in the provided box. a united nations toxicologist studying the properties of must...
Questions

Biology, 16.09.2019 01:30

Computers and Technology, 16.09.2019 01:30




Mathematics, 16.09.2019 01:30

History, 16.09.2019 01:30

Biology, 16.09.2019 01:30

Mathematics, 16.09.2019 01:30


Mathematics, 16.09.2019 01:30





History, 16.09.2019 01:30

Spanish, 16.09.2019 01:30

Mathematics, 16.09.2019 01:30

Physics, 16.09.2019 01:30

Biology, 16.09.2019 01:30

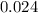
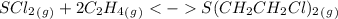
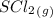 = 0.325 M
= 0.325 M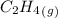 = 0.273 M
= 0.273 M 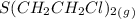 = 0.35 M
= 0.35 M![Kc=\frac{[S(CH_2CH_2Cl)_2]}{[SCl_2][C_2H_4]^2}=\frac{[0.35]}{[0.325][0.273]^2}=14.45](/tpl/images/0341/2750/de285.png)
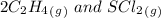 = -2
= -2